A Look at 3 of the Approved COVID-19 Vaccines
The second wave of the COVID-19 pandemic has been as drastic as expected: worldwide deaths per day, on average, have risen sharply since October. There were around 5,000 deaths per day in early October, but that number reached 15,124 on December 30. Moreover, the novel coronavirus had killed more than 1.8 million people as of early January, and there had been more than 85 million recorded cases.
Fortunately, two vaccines have been approved in the US and seven in total have been authorized for use by various governments worldwide. Below is a look at three of the seven approved COVID-19 vaccines.
Pfizer BNT162b2
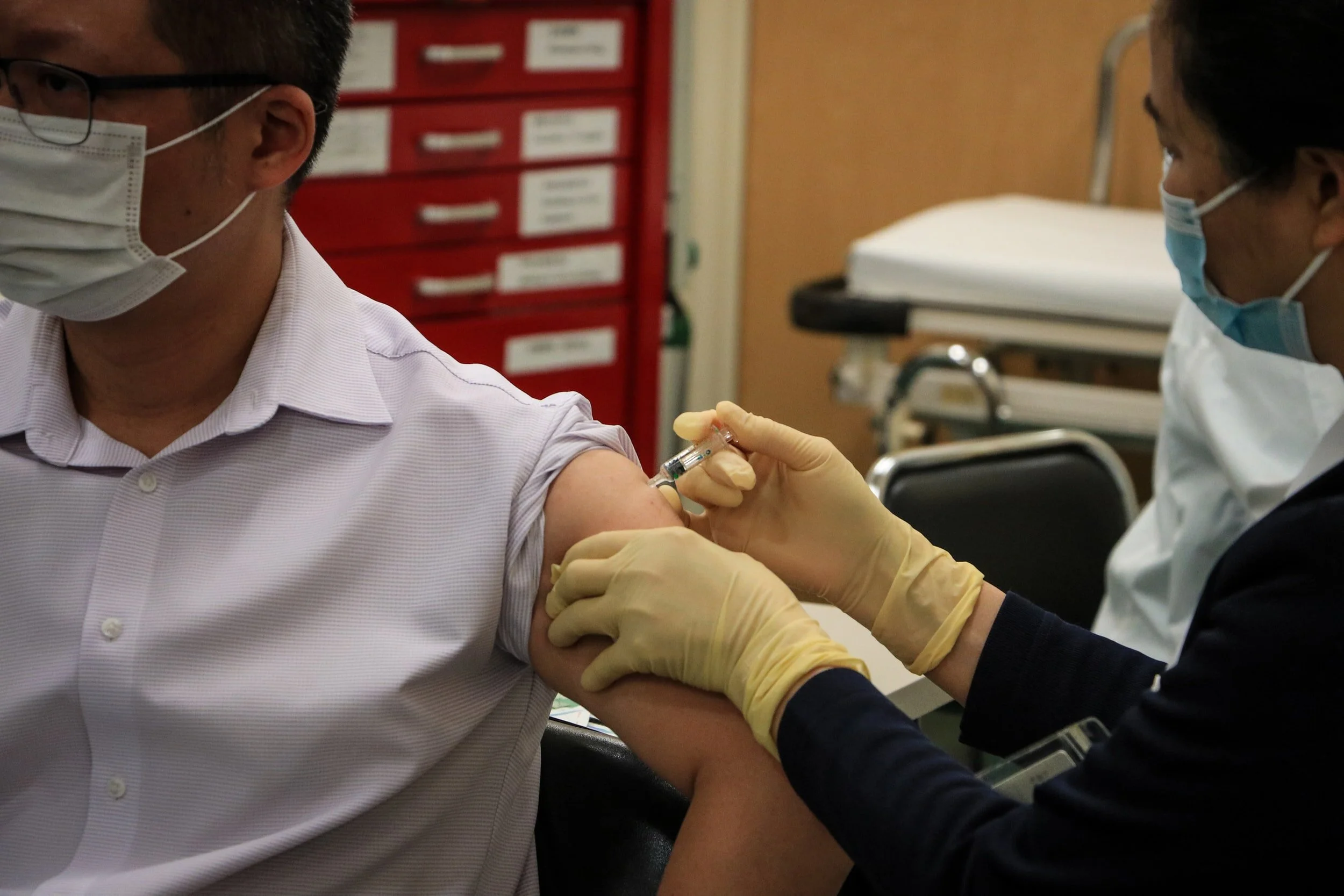
Developed by Pfizer and BioNTech, the BNT162b2 vaccine is the most widely-approved COVID-19 vaccine worldwide. It was first granted temporary authorization by the United Kingdom on December 2 and has since been granted approval in Bahrain, Canada, Mexico, Chile, and the US, among other countries. It works by encoding a mutated version of the SARS-CoV-2 to generate an immune response against the virus.
The vaccine is injected into a muscle in the upper arm of recipients through two shots occurring three weeks apart. Side effects can occur within a couple days of receiving the vaccine and may include pain and swelling around the arm as well as fatigue, chills, and headaches. These are more common following the second dose. BNT162b2 was 95 percent effective in clinical trials, which is an "extraordinarily high degree of efficacy," according to Dr. Anthony Fauci.
Moderna mRNA-1273
The Moderna mRNA-1273 vaccine is one of only three vaccines to be authorized for use in multiple countries. Approved in both the US and Canada, among other countries, the two-dose vaccine is given four weeks apart. It was funded by Operation Warp Speed and has received support from other partners including Emory University and the Dolly Parton COVID-19 Research Fund.
The US ordered 200 million doses of the mRNA-1273 vaccine and was expected to deliver 20 million doses by the end of 2020. The European Commission has ordered 160 million doses, while countries including Canada, Japan, and Switzerland ordered millions before the end of 2020.
Beijing Institute of Biological Products (BBIBP) CorV
One of three vaccines developed in China, the BBIBP-CorV inactivated vaccine was first approved for use by essential personnel and healthcare workers in the country following encouraging results in Phase 1 and 2 trials. Phase 3 trials are ongoing in China and Argentina. The vaccine has also been approved for use in Bahrain and the United Arab Emirates, with the latter announcing it was 86 percent effective.